D.med Engineering
Engineering solutions for a healthier world
We bring your medical device ideas to life with expertise, excellence, and an outstanding motivation.
About D.med Engineering
Experienced with innovative mindset
D.med Engineering is our business unit that revolves around the development of medical products from the first idea to the final market launch. Under the roof of D.med Engineering we combine world-class expertise in medical engineering, software development, manufacturing, medical and regulatory affairs, quality assurance and project management, to successfully realize your project.
D.med Engineering Entities
D.med Engineering comprises various specialized business units, each with over a decade of expertise in their respective fields. Among these entities, D.med Consulting (DMC) stands as the backbone, leading and driving projects within the business unit.

Our Services
From the initial idea to the finished product
Software Solutions
- Embedded software development
- Cloud and web/desktop application software
- Cybersecurity for medical devices
Electronic Engineering
- Electronic development
- Design and prototyping
- Structural analysis and simulation
Manufacturing and Quality Control
- Design for manufacturability
- Quality control and regulatory compliance
- Supply chain management
R&D and Innovation
- Research and development of extracorporeal medical devices
- Innovation consulting and product ideation
- User research and usability

References
Selected Projects

Fresenius 5008S CorDiax and 6008 CAREsystem
Fresenius 5008S CorDiax and 6008 CAREsystem
The development team of D.Med Consulting has played a crucial role in various projects for Fresenius Medical Care, leading to the successful development of dialysis machines like the 5008S CorDiax and the 6008 CAREsystem.

D.Med Technical System (DTS)
D.Med Technical System (DTS)
DTS is a service software designed to monitor medical devices, interventions (such as repairs, maintenance, and safety inspections), customers, and measurement tools. Featuring a user-friendly interface and a logical workflow, DTS ensures intuitive handling, simplifying administrative processes, documentation, and technical surveillance.

NephroFlow
NephroFlow
In collaboration with emtec GmbH, a German technology leader in non-invasive flow measurement, D.Med Consulting has developed NephroFlow, an advanced access-flow and recirculation measurement system tailored for dialysis.

Regulatory Affairs Consulting
Regulatory Affairs Consulting
With its regulatory affairs expertise, D.Med Consulting successfully supported a leading dialysis machine manufacturer in adapting their class 2b product to comply with the new Medical Device Regulation (MDR). Our services also include Gap Assessment, technical file updates, normative tests management, clinical evaluation, and communication with notified bodies.

Nipro Surdial X Haemodialysis Machine
Nipro Surdial X Haemodialysis Machine
The Nipro Surdial-X, a high-end hemodialysis machine, is proudly developed by D.Med Consulting. Our expertise in delivering state-of-the-art technology has enabled Nipro Corporation Japan to introduce this high-end product to the global market, advancing the field of hemodialysis.

Monitoring & Database Software for Dialysis Clinics
Monitoring & Database Software for Dialysis Clinics
We proudly present our medical software solution for efficient management of patient data and dialysis prescriptions. This software empowers users to store and access patient-related information and dialysis prescriptions in a smart database.

Cost-Down Technical Assessment
Cost-Down Technical Assessment
D.Med Software's Cost-Down Technical Assessment project aims to identify savings for enhanced device affordability, while exploring new technologies for improved effectiveness and reduced costs. Our approach includes an initial analysis, redesigned PCB development, software revision, and new test software creation to streamline production and increase accessibility.

Ex Vivo Lung Perfusion (EVLP)
Ex Vivo Lung Perfusion (EVLP)
In collaboration with a renowned manufacturer of Ex Vivo Lung Perfusion (EVLP), D.Med Consulting successfully developed a concept for the next generation of EVLP devices aiming to reduce the cost per treatment and meet the growing demand for transplantable lungs, thereby enhancing the quality of life for patients.
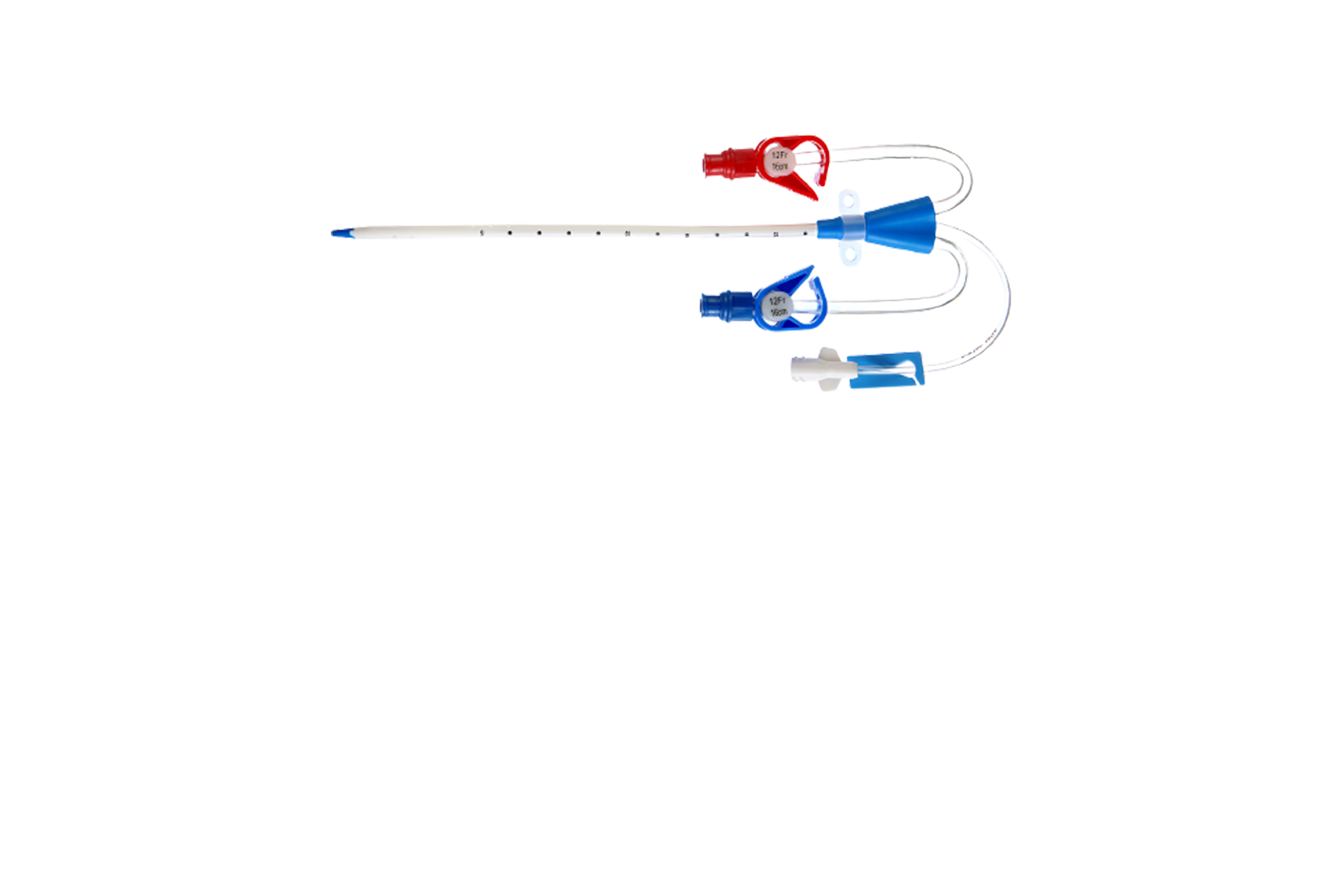
Long-Term Dialysis Catheter Test Rig and Optimization Project
Long-Term Dialysis Catheter Test Rig and Optimization Project
In this project, we focused on optimizing the positioning of long-term dialysis catheters. It involved the utilization of a specialized test rig to measure flow and recirculation rates at different catheter positions. The gathered data resulted in improved efficiency and enhanced patient comfort.
We are Certified
D.med Consulting: The Backbone of Quality Management at D.med Engineering.
Our commitment to quality is evident through the certification of D.med Consulting GmbH (DMC), our dedicated project management entity within the D.med Engineering business unit. D.med Engineering’s Commitment to Excellence, DMC has obtained certification for its advanced quality management system in accordance with the DIN EN ISO 13485:2016 standards.
With over a decade of specialized expertise in quality management, DMC serves as the backbone of our operations at D.med Engineering, ensuring that we consistently meet the highest standards of excellence.

Contact Us
Connect with us for collaborative opportunities

Andreas Happold
Director Business Development
With a deep passion for the newest technology, we provide our customers the optimal solution for their medical product realization from the first assessment to series production. According to international standards we find the most effective way to market entry.
We are looking forward to starting a trustful cooperation with you!








