About Us
Experienced with innovative mindset
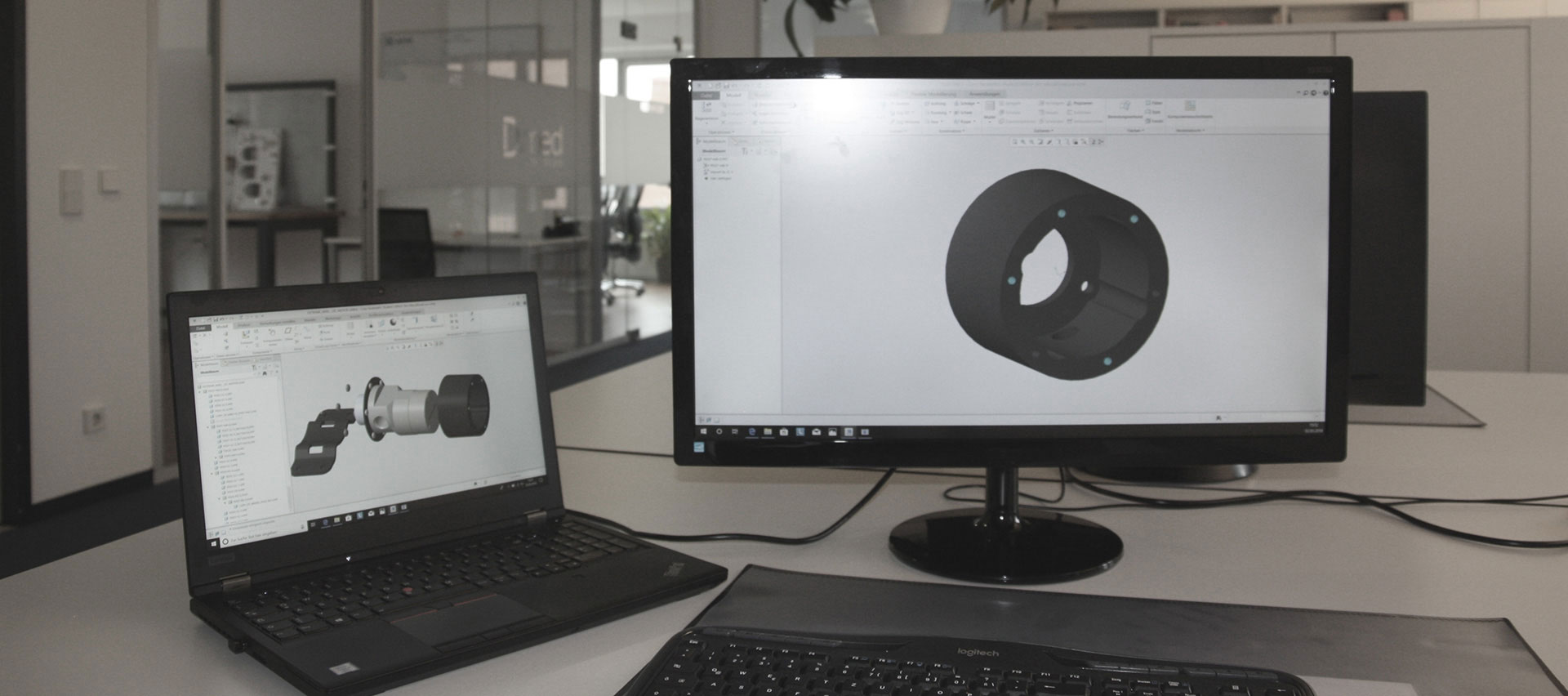
D.Med Consulting GmbH is a leading specialist in extracorporeal technologies and other related medical fields. From the initial idea to the finished product, we develop new suitable solutions, re-engineer existing products, and prepare your market launch. We do not work in a preset structure but develop tailored solutions in cooperation with our clients.
D.Med Consulting GmbH was founded in 2011 and is based in Hamburg. Since 2019 it is a joint venture of Fresenius Medical Care, the global market leader in the field of dialysis, and D.med Healthcare Group, a global provider of medical services and medical products in nephrology, diabetes and other fields of internal medicine.
Our Services
From the initial idea to the finished product
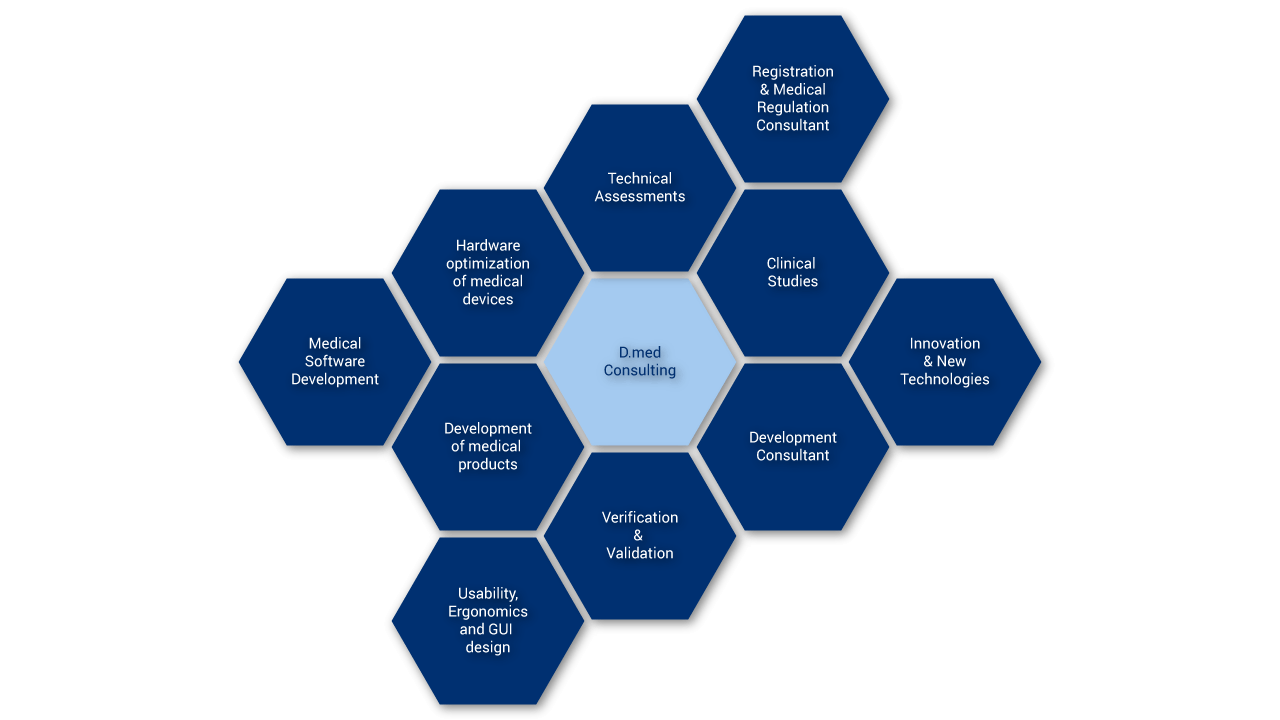
Development of medical products
Based on your expectations and our expertise, we develop your product from the initial idea to the final registration.
Verification and Validation
An important part of product development deals with the extensive testing of the medical device for its heart and kidneys (German figure of speech: put sth. to the acid test). Together with you, we develop suitable test strategies for the verification and validation of your medical product. This includes, among other things, analyses of test basis, acceptance criteria and sample size. We accompany you from test planning to test execution and reporting of test results – always with the goal of proving the quality of your medical device.
Clinical Studies
D.med Consulting’s dedicated team includes experts in the field of medical practice and clinical studies. We develop study designs and support you in managing and monitoring the studies in an organized way.
Development Consultant
If you have your own development department, we can offer to consult you in all aspects of your development projects. Based on our extensive market and regulatory know-how we can support you in the definition of suitable user requirements and guide you through a slim and effective development and registration process.
Registration and medical regulation consultant
Regardless of whether you need support with the approval of a medical device, the fulfillment of national or international regulations, or communication with the notified body, D.Med Consulting GmbH is at your side and provides you with comprehensive support. Furthermore, we are experts in quality management-related aspects and can assist you to establish a quality management system such as ISO 13485 in your company.
Modern hardware optimization of medical devices
We optimize your hardware including electric systems under economical and ecological aspects. With our vast knowledge of the component market, we reduce your production costs by selecting the right suppliers with you and support you in “make-or-buy” decisions.
Usability, Product Ergonomics and Graphical User Interface Design
The success of any medical device is increasingly more dependent on modern design and excellent usability. We assist you in optimizing this important aspect and increasing the safety of your product. With usability engineering, we ensure that your product is user-friendly and that potential user errors will not lead to device failures. We gather feedback directly from the target audience to optimize the product and improve user retention.
With our knowledge of applicable standards such as IEC 60601-1-6 or IEC 62366, we are also able to create usability files for you.
Medical Software Development
We guarantee reliable and high assurance Medical Software Development in accordance with IEC-62304. Additionally, we also support in software documentation according to the standard requirements and support in cybersecurity projects.
Technical Assessment
With our advanced understanding of medical devices, we are your perfect partner to analyze your machine or developed prototype. We provide assessments of the hardware concept, software architecture, functional safety, usability, graphical-user-interface, instruction of use and service manual. Additionally, we present extensive and detailed reports based on the results of our SWOT analysis.
References
Some selected projects
Nipro
Surdial X
As the lead developer of the Nipro Surdial-X, we enabled Nipro Corporation Japan to introduce a high-end, state-of-the-art hemodialysis machine to the international market and developed optional features like Kt/V measurement, blood volume measurement, monitoring, optimized substitution flow control, etc. Tradename/Product of Nipro Corporation® Japan
D.med
NephroFlow
The D.med NephroFlow, an advanced access-flow, and recirculation measurement system, is the latest product of D.med Healthcare in the field of dialysis. It was developed in close cooperation with German-based emtec GmbH, a technology leader in non-invasive flow measurement.
D.med Technical
System (DTS)
DTS is a service software developed by D.med to track your medical devices, interventions (e.g. repairs, maintenance, and safety inspections), customers, and measurement tools. With its user-friendly interface and a logical workflow that enables intuitive handling, DTS simplifies administrative processes, documentation, and technical surveillance.
Guidance & consulting
for new MDR
We support customers in adapting to the new Medical Device Regulation (MDR). That support includes the modification of any documentation and technical files as required. Additionally, we can consult you in the execution of new required processes.
Monitoring & Database
Software for Dialysis Clinics
Development of medical software to manage patient data and dialysis prescriptions. This software allows users to store patient-related data and dialysis prescriptions in a smart database enabling the clinic staff to monitor parameters in real time during dialysis treatment. Additionally, the software tracks laboratory results, vital parameters, and medications.
Fresenius
5008S and 6008
D.med Consulting was and is still involved in multiple Fresenius Medical Care projects for the dialysis machines 5008S and 6008.
Focus on quality
We are Certified


Advanced quality management is the key driver to guarantee outstanding treatment results independent of local reimbursement rates. That is why D.Med Consulting GmbH shows a certified quality management system according to DIN EN ISO 13485:2016 with the scope Contract Design and Development of Medical Devices for Extracorporeal Treatment of Blood. This proves that all our development-related projects and their supporting processes are covered by the certificate and fulfill a globally valid Quality Standard.